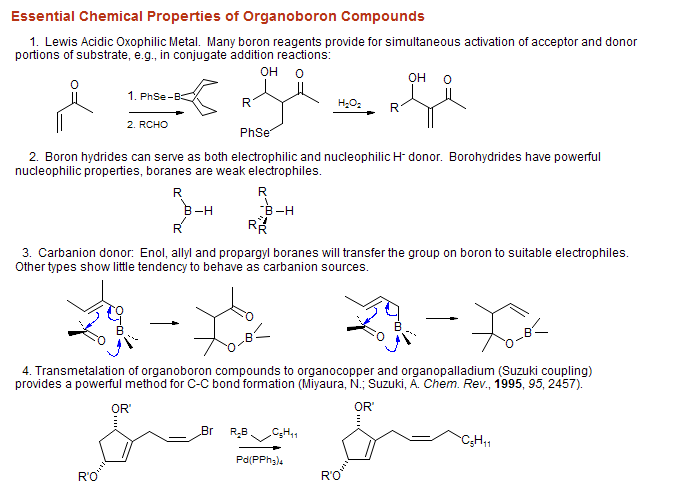
Boron in Organic Synthesis
© Hans J. Reich 2008
Organoboron and other C-C bond forming reactions in some representative syntheses: The structures below are labeled with the organometal used to form the indicated C-C bonds. Note that organoboranes are largely used to couple carbons with one or two adjacent stereocenters. B = organoboron reagent, Li = lithium reagent, Mg = Grignard reagent, Cu = organocopper reagent, P = Wittig reagent, Li/P Na/P K/P Horner-Wadsworth-Emmons, Pd/Sn = Stille coupling, Pd/Zn = Negishi coupling, Li/Si = Peterson olefination, Zr/Al = Tebbe reagent, R = Radical addition/cyclization.

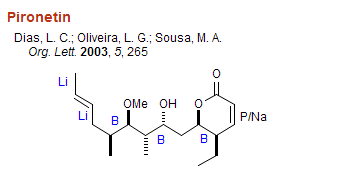
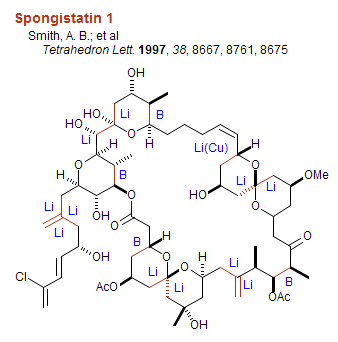
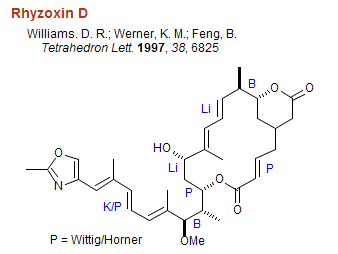
Organoboron Reviews
Organoborates in New Synthetic Reactions,
Suzuki, A. Acc. Chem. Res. 1982, 15, 178; Top. in Current Chem. 1983, 112.
Carbon-Carbon Formation Involving Boron Reagents,
A. Pelter Chem. Soc. Rev. 1982, 11, 191.
Formation of Carbon-Carbon and Carbon-Heteroatom Bonds via Organoboranes and Organoborates,
E.-I. Negishi, M. J. Idacavage Org. React. 1985, 33, 1.
Organoboron Compounds in Organic Synthesis,
R. M. Mikhailov, Harwood Academic, 1984.
Reactions of Group 13 Alkyls with Dioxygen and Elemental Chalcogens: from Carelessness to Chemistry,
Barron, A. R. Chem. Soc. Rev. 1993, 22, 93.
Stereodirected Synthesis with Organoboranes,
Trost, B.M. Ed., Springer: Berlin, Germany, 1995.
Contemporary Boron Chemistry,
Davidson, M.; Hughes, A. K.; Marder, T. B.; Wade, K. Royal Society of Chemistry: Cambridge, U.K., 2000.
Rhodium-Catalyzed Asymmetric 1,4-Addition of Organoboronic Acids and Their Derivatives to Electron Deficient Olefins.
Hayashi, T. Synlett 2001, 879-87.
Pure Enantiomers via Chiral Organoboranes,
H. C. Brown, B. Singram Accounts Chem. Res. 1988, 21, 287.
Boronic Esters in Stereodirected Synthesis,
D. S. Matteson Tetrahedron 1989, 45, 1859.
Recent Advances in Asymmetric Synthesis with Boronic Esters,
Matteson, D. S. Pure & Appl. Chem. 1991, 63, 339.
Stereodirected Synthesis with Organoboranes,
D. S. Matteson, Springer, 1995.
Asymmetric Syntheses via Chiral Organoboranes Based on α-Pinene,
by Brown, H.C. Adv. in Asymm. Synth. Vol. 1, Hassner, A., Ed. JAI: Greenwich, CT, 1995.
α-Halo Boronic Esters in Asymmetric Synthesis,
Matteson, D. S. Tetrahedron 1998, 54, 10555-607.
Vinyl Boranes:
Synthetic Applications of Vinylic Organoboranes,
H. C. Brown and J. B. Campbell, Jr. Aldrichim. Acta 1981, 14, 3.
Haloboration of 1-Alkynes and Its Synthetic Application [Vinyl Boranes],
Suzuki, A. Rev. Heteroatom Chem. 1997, 17, 271-314.
Recent Developments in the Chemistry of Amine- and Phosphine-Boranes,
Carboni, B.; Monnier, L. Tetrahedron 1999, 55, 1197-248.
Useful Synthetic Transformations Via Organoboranes. 1. Amination Reactions,
Carboni, B.; Vaultier, M. Bull. Soc. Chim. Fr. 1995, 132, 1003-8.